õĖŁÕøĮń¤Žxča(b©│)Õż¦ÕŁ”ÕQłÕīŚõ║¼’╝ēńÄŗķøģÕÉøŃĆüµĖģÕŹÄÕż¦ÕŁ”µ£▒µ░Ėµ│ĢŃĆŖChem. Eng. J.ŃĆ? ķĆÜĶ┐ćĶ░āĶŖéPtńÜäÕī¢ÕŁ”ńŖȵĆüõ┐āśqøÕģēÕé¼Õī¢ÕłåĶ¦Żµ░┤õ±öµ░?/span>
2022-05-03 µØźµ║É:õĖŁÕøĮĶüÜÕÉłńē®ńĮæ ńé╣Õć╗ŗŲ?/span>
Õģ│ķö«Ķ»Ź’╝Ü(x©¼)ń¤ø_ó©ńøĖµÉ█Õī¢ńó│ŃĆüPt0ńē®ń¦ŹŃĆüÕĤõĮŹń║óÕż¢ŃĆüÕģēÕé¼Õī¢õ║¦µ░ó
Ķ┤ĄķćæÕ▒?span style="font-family:;">PtÕøĀÕģČÕģõh£ēÕÉłķĆéńÜäĶ┤╣ń▒│ĶāĮń±öĶĆīĶó½ńö©õĮ£ÕĖĖńö©ńÜäÕŖ®Õé¼Õī¢Õēé’╝īÕģõh£ēŗz└LƦķ½śŃĆüń©│Õ«ÜµĆ¦ÕźĮĮ{ēõ╝śÕŖčØĆéõĮ£õĖ║ÕŖ®Õé¼Õī¢Õēé’╝īPtńÜäÕī¢ÕŁ”ńŖȵĆüÕ»╣ÕģēÕé¼Õī¢ÕēéńÜäµ×ɵ░óµ┤╗µĆ¦µ£ēµśŠĶæŚÕĮ▒ÕōŹÕQīõĮåśqÖń¦ŹÕĮ▒ÕōŹ×«Üµ£¬ÕŠŚÕł░µĘ▒ÕģźńĀöń®ČŃĆéµø┤õĖ║ķćŹĶ”üńÜ䵜»’╝īõ║åĶ¦ŻPtÕ£©ÕģēÕé¼Õī¢ÕÅŹÕ║öśqćń©ŗõĖŁńÜäń£¤Õ«×µĆ¦Ķ┤©ÕQīÕÅ»õ╗źõžō(f©┤)µ×äÕŠÅÕģõh£ēķ½śµ┤╗µĆ¦ńÜäÕģēÕé¼Õī¢ÕēéµÅÉõŠøµ¢░ńÜäµĆØĶĄ\ŃĆ?/span> śqæµ£¤ÕQīõĖŁÕøĮń¤│µ▓╣Õż¦ÕŁ”’╝łÕīŚõ║¼ÕQēńÄŗķøģÕÉøÕē»ńĀöĮIČÕæśĶ»∙NóśŠläõĖĵĖģÕŹÄÕż¦ÕŁ”µ£▒µ░Ėµ│ĢµĢÖµÄłÕ£©ŃĆ?/span>Chemical Engineering JournalŃĆ?/span>µ£¤ÕłŖõĖŖÕÅæĶĪ©õ║åķóśõžō(f©┤)ŌĆ?/span>Boosting photocatalytic hydrogen evolution via regulating Pt chemical statesŌĆØńÜäµ¢ćń½ĀÕQ?span style="font-family:;">DOIÕQ?/span>10.1016/j.cej.2022.136334ÕQēŃĆéµ£¼Ķ«║µ¢ćµÅÉÕć║õ║åõĖĆ┐UŹµ£ēµĢłńÜäĮ{¢ńĢźÕQīķĆÜĶ┐ćĶ░āĶŖéPtńÜäÕī¢ÕŁ”ńŖȵĆüµØźÕż¦Õ╣ģÕ║”µÅÉķ½?/span>Pt/g-C3N4ÕģēÕé¼Õī¢ÕēéńÜäõ±öµ░óµĆ¦ĶāĮŃĆéµ¢ćõĖŁÕłČÕżćõ║åõĖŹÕÉīPt0ÕɽķćÅńÜ?/span>Pt/g-C3N4Õé¼Õī¢Õēé’╝īÕÅæńÄ░µÅÉķ½śPt0ÕɽķćÅÕÅ»õ╗źÕż¦Õ╣ģµÅÉķ½śÕģēĶ¦Żµ░┤õ±öµ░óµ┤╗µĆ¦ŃĆéÕĤõĮŹń║óÕż¢ÕģēĶ░▒ÕÆīDFTńÉåĶ«║Ķ«Īń«ŚĶ»üµśÄÕQīµ░öµ░?/span>ÕżäńÉåõĮ┐ńöĄ(sh©┤)ÕŁÉõ╗Äg-C3N4ńÜ?/span>NÕÄ¤ÕŁÉĶĮ¼ń¦╗Õł?/span>Pt2 õĖ?/span>ÕQīõ╗ÄĶĆīÕó×ÕŖĀõ║åPt0ńē®ń¦ŹńÜäµĢ░ķćÅŃĆ?/span>Pt0ńē®ń¦ŹńÜäÕż¦ķćÅńö¤µłÉµ£ēÕł®õ║ÄÕŖĀķƤÕģēńö¤ńöĄ(sh©┤)ĶŹ’L(f©źng)ÜäÕłåń”╗ŃĆ鵣żÕż¢’╝īPt0µ»?/span>Pt2 Õģõh£ēµø┤õĮÄńÜäµ░óµ░öÕÉĖķÖäĶāĮÕQīµ£ēÕł®õ║ĵ░óµ░öńÜäµ║óÕć║ŃĆéÕøĀµŁż’╝īÕģõh£ēķ½śµ»öõŠ?/span>Pt0ńÜäÕģēÕé¼Õī¢ÕēéÕģʵ£ēµø┤ķ½śńÜäõ║¦µ░óŗz└LƦŃĆ?/span>
µ£¼µ¢ćķććńö©õ║åķ½śµĖ®ńāŁĶüÜÕÉłŃĆüĶČģÕŻ░µ╣┐ŗ╣ĖµĖŹÕQ?/span>1.0%-Pt/CNÕQēŃĆüÕģēµ▓ēń¦»ÕQ?/span>1.0%-Pt/CN-PÕQēŃĆ?/span>NaBH4µČ▓ńøĖśqśÕĤÕQ?/span>1.0%-Pt/CN-BHÕQēÕÆīµ░óµÉ█µĘĘÕÉłµ░öńāŁÕżäńÉåÕQ?/span>1.0%-Pt/CN-BH-HÕQēńŁēµ¢ęÄ(gu©®)│ĢÕłČÕżćõ║?/span>Pt/g-C3N4ÕżŹÕÉłµØɵ¢ÖŃĆéķĆÜĶ┐ćXPSĶĪ©ÕŠüÕÅæńÄ░PtõĖ╗Ķ”üõ╗?/span>Pt0ńē®ń¦ŹńÜäÕ┼×Õ╝ÅÕŁśÕ£©õ║Ä1.0%-Pt/CN-BH-HµĀĘÕōüõĖŁŃĆéńøĖÕÅŹ’╝īPtõĖ╗Ķ”üõ╗?/span>Pt2 ÕĮóÕ╝ÅÕŁśÕ£©õ║?/span>1.0%-Pt/CN-PÕÆ?/span>1.0%-Pt/CN-BHµĀĘÕōüõĖŁŃĆéń╗Åśqćµ░óµ░«µž£ÕÉłµ░öµ░øÕżäńÉåÕÉÄÕQ?/span>PtńÜäÕī¢ÕŁ”ńŖȵĆüÕÅæńö¤µśŠĶæŚÕÅśÕī¢’╝ī1.0%-Pt/CN-BH-HõĖ?/span>Pt0ńÜäµ»öõŠŗµśŠĶæŚÕó×ÕŖĀŃĆ?/span>1.0%-Pt/CN-P, 1.0%-Pt/CN-BHÕÆ?1.0%-Pt/CN-BH-HõĖ?/span>Pt0ńÜäµ»öõŠŗÕłåÕł½õžō(f©┤)8.2%ÕQ?/span>10.0%ÕÆ?/span>60.1%ÕQłÕøŠ1cÕQēŃĆéń╗Åśqćµ░öµ░øÕżäńÉåÕÉÄÕQ?/span>NÕģāń┤ĀÕÉæķ½śŠlōÕÉłĶāĮµ¢╣ÕÉæń¦╗ÕŖ©’╝īPtÕģāń┤ĀÕÉæõĮÄŠlōÕÉłĶāĮµ¢╣ÕÉæń¦╗ÕŖ©’╝īĶĪ©µśÄńö?sh©┤)ÕŁÉõ?/span>NÕÉ?/span>Pt0ĶĮ¼ń¦╗ÕQłÕøŠ1bÕQ?/span>ŃĆéĶ┐Öõ║øń╗ōµ×£ĶĪ©µśÄ’╝ī1.0%-Pt/CN-BH-HõĖŁÕɽµ£ēÕż¦ķćÅńÜäPt0ńē®ń¦Źµ£ēÕł®õ║ÄńöĄ(sh©┤)ĶŹ’L(f©źng)ÜäÕłåń”╗ÕÆīĶØ{┐UģRĆ?/span>ķĆÜĶ┐ćCOÕÉöRÖäŠUóÕż¢ÕģēĶ░▒ÕQłÕøŠ1dÕQēÕŠŚÕć?/span>2115 cm-1ÕżäńÜäÕÉöRÖäÕ│?/span>õĖ?/span>COÕ£?/span>Pt2 ńē®ń¦ŹõĖŖńÜäÕÉöRÖäŃĆ?/span>2055 cm-1ÕżäńÜäÕÉöRÖäÕ│?/span>õĖ?/span>COÕ£?/span>Pt0ńē®ń¦ŹõĖŖńÜäŠU┐µĆ¦ÕÉĖķÖäŃĆ?/span>ŠlÅĶ┐ćµ░öµ░øÕżäńÉåÕÉÄ’╝īPt0ńē®ń¦ŹńÜäµ»öõŠŗµśŠĶæŚÕó×ÕŖĀŃĆéĶ┐ÖõĖĆŠlōĶ«║õĖ?/span>XPSŠlōµ×£õ┐صīüõĖĆĶć?/span>ŃĆ?/span>
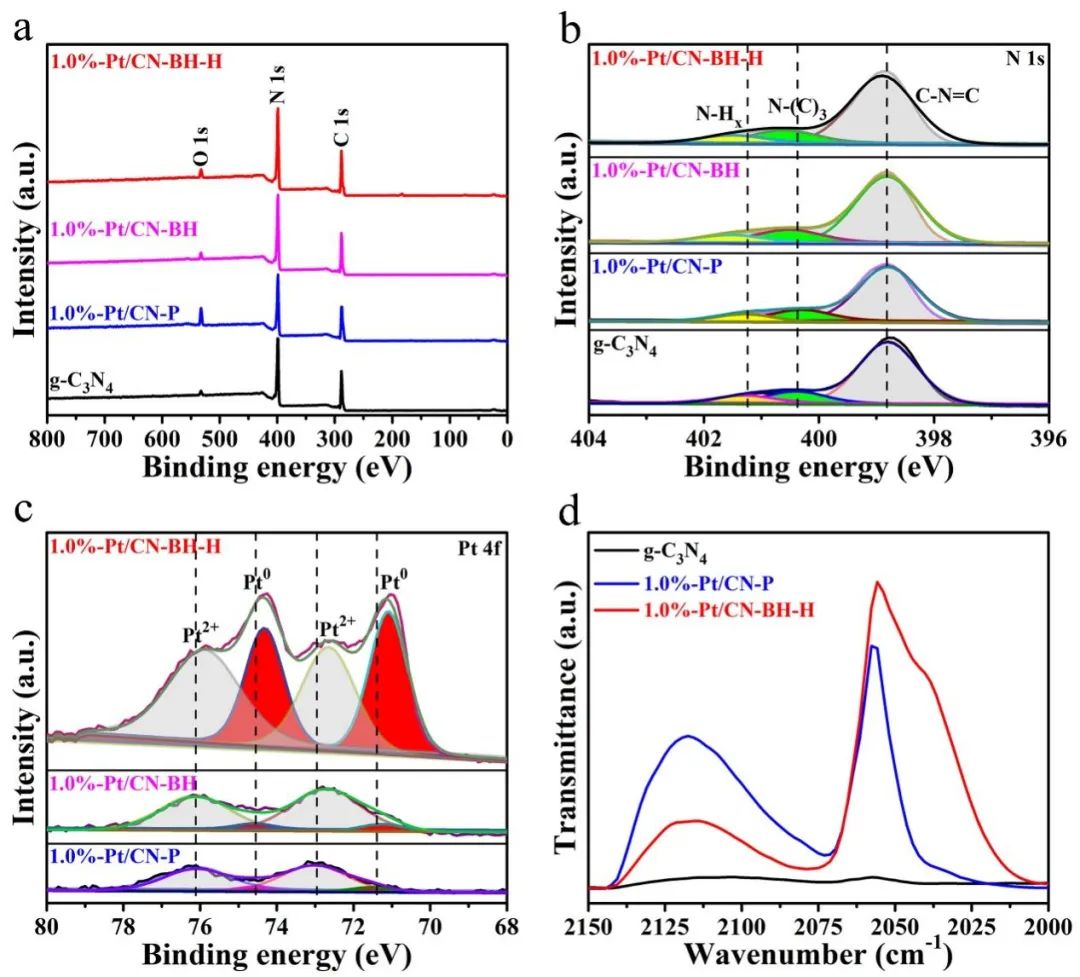
Õø?/span>1 ÕQ?/span>aÕQ?/span>XPSÕģ©Ķ░▒ŃĆ?/span>µĀĘÕōüńÜ?/span>N 1sÕQ?/span>bÕQēÕÆīPt 4fÕQ?/span>cÕQēÕģāń┤ĀĶ░▒ŃĆ?/span>ÕQ?/span>dÕQēµĀĘÕōüńÜäCOÕÉöRÖäŠUóÕż¢ÕģēĶ░▒ŃĆ?/span>
Õ£©ÕÅ»Ķ¦üÕģēµØĪõÜgõĖŗ’╝ī1.0 %-Pt/CN-BH-H µĀĘÕōüÕQ?/span>Pt0ńÜäµ»öõŠŗõžō(f©┤)60.1%ÕQ?/span>Õģõh£ēµ£Ćķ½śńÜäÕģēÕé¼Õī¢µ┤╗µĆ?/span>ÕQ?/span>2.316 mmol h-1 g-1ÕQ?/span>ÕQīń║”õĖ?/span>1.0 %-Pt/CN-P µĀĘÕōüÕQ?/span>0.605 mmol h-1 g-1 , Pt0ńÜäµ»öõŠŗõžō(f©┤)8.2%ÕQ?/span>ÕÆ?/span>1.0%-Pt/CN-BHµĀĘÕōüÕQ?/span>0.644 mmol h-1 g-1 , Pt0ńÜäµ»öõŠŗõžō(f©┤)10.0%ÕQ?/span>ńÜ?/span>4ÕĆ?/span>ÕQłÕøŠ2aÕQ?/span>ŃĆéÕøĀµŁż’╝īÕé¼Õī¢ŗz└LƦõĖÄPtńē®ń¦ŹńÜäÕī¢ÕŁ”ńŖȵĆüµ£ēÕģ¤ļĆ?/span>µłæõ╗¼ÕÅ»õ╗źĶ«żõžō(f©┤)ÕQ?/span>1.0%-Pt/CN-BH-HõĖ?/span>Pt0ńÜäµ»öõŠ?/span>ŁæŖķ½śÕQīÕģēÕé¼Õī¢µĆ¦ĶāĮŁæŖÕźĮŃĆéķĆÜĶ┐ćÕżÜµ¼ĪÕŠ¬ńĻի×ķ¬īĶĪ©µśÄ1.0%-Pt/CN-BH-HÕģõh£ēõ╝śÕ╝éńÜäń©│իܵĆ?/span>ÕQłÕøŠ2bÕQēŃĆ?/span>1.0%-Pt/CN-BH-HÕ£?/span>420 nmõĖŗńÜäĶĪ©Ķ¦éķćÅÕŁÉµĢłńÄćõĖ?/span>8.1%ÕQīµśŠĶæŚķ½śõ║?/span>1.0%-Pt/CN-PÕQ?/span>4.0%ÕQ?/span>ÕQīĶ»┤µśÄÕēŹĶĆģÕ£©ÕÅ»Ķ¦üÕģēµØĪõ╗ČõĖŗÕģõh£ēõ╝śĶČŖńÜäµ┤╗µĆ?/span>ÕQłÕøŠ2cÕQ?/span>ŃĆ?/span>
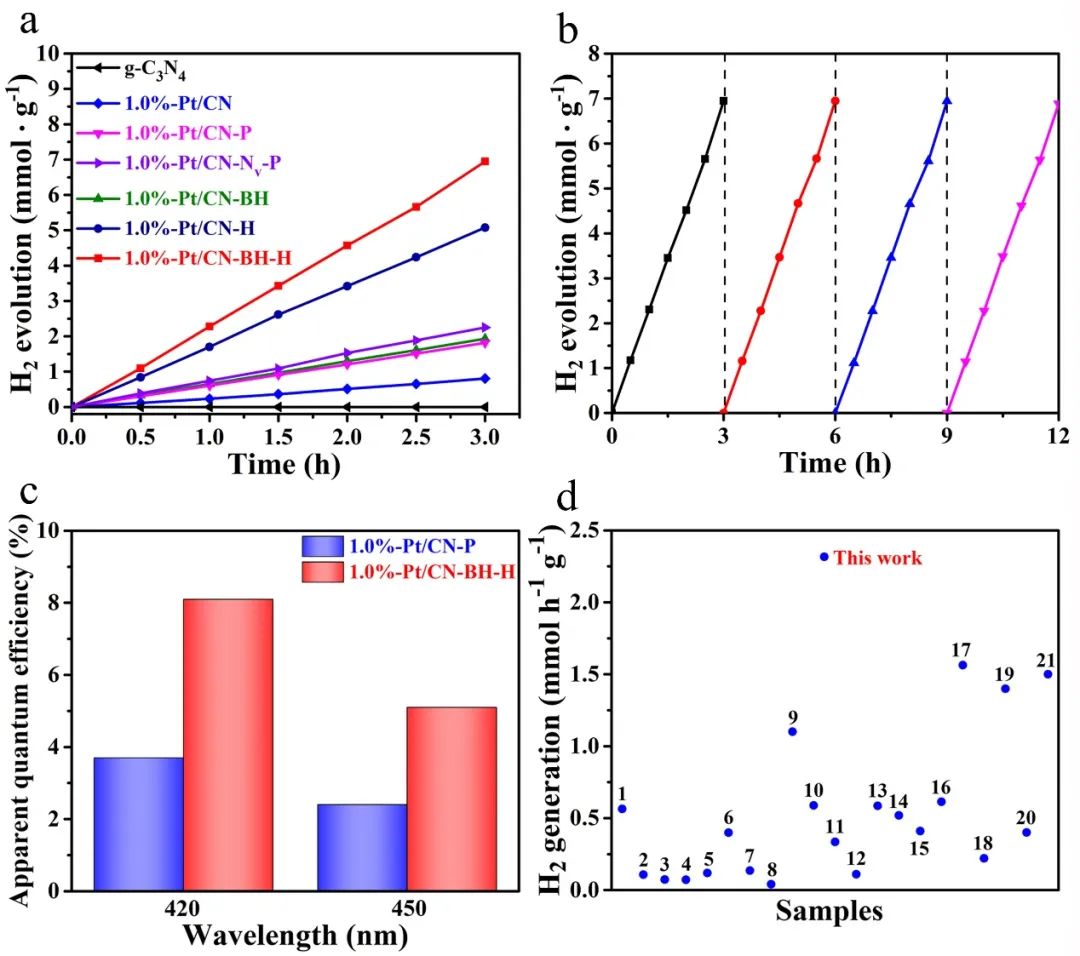
Õø?/span>2 ŗz└LƦµĄŗĶ»ĢŃĆé’╝łaÕQēµĀĘÕōüÕ£©ÕÅ»Ķ¦üÕģē’╝ł╬╗ Ōē?420 nmÕQēõĖŗńÜäÕģēÕé¼Õī¢õ║¦µ░óķƤńÄćŃĆé’╝łbÕQ?/span>1.0%-Pt/CN- BH-HńÜäÕģēÕé¼Õī¢õ║¦µ░óÕŠ¬ńĻի×ķ¬īŃĆé’╝łcÕQ?/span>1.0%-Pt/CN-PÕÆ?/span>1.0%-Pt/CN-BH-HÕ£?/span>420ÕÆ?/span>450 nmÕżäńÜäÕŹĢµćLķĢ┐ĶĪ©Ķ¦éķćÅÕŁÉµĢłńÄćŃĆ?/span>ÕQ?/span>dÕQēõĖŹÕÉ?/span>Pt/g-C3N4ÕżŹÕÉłµØɵ¢ÖÕģēÕé¼Õī¢õ±öµ░óķƤńÄćńÜäµ»öĶŠāŃĆ?/span>
õĖ▐Z║åńĀöń®ČõĖŹÕÉīPtńē®ń¦ŹÕQ?/span>Pt2 ÕÆ?/span>Pt0ÕQ?/span>õĖ?/span>g-C3N4õ╣ŗķŚ┤ńÜäńöĄ(sh©┤)ĶŹĘÕłå╝ø└LĢłńÄć’╝īµłæõ╗¼ŗ╣ŗķćÅõ║?/span>1.0%-Pt/CN-BH-HÕÆ?/span>1.0%-Pt/CN-PÕ£©ÕÅ»Ķ¦üÕģēµØĪõÜgõĖŗńÜäÕĤõĮŹŠUóÕż¢ÕģēĶ░▒ŃĆ?/span>õĖ?/span>1.0%-Pt/CN-BH-HÕ£©ķ╗æµÜŚµØĪõ╗ČõĖŗńøĖÕ»╣ĶŠāõĮÄńÜ?/span>Õ│?/span>Õ╝║ńøĖµ»ö’╝īķÜÅńØĆÕģēńģ¦µŚēÖŚ┤ńÜäÕó×ÕŖĀ’╝īńē╣ÕŠüÕ│░µśŠĶæŚµÅÉķ½?/span>ÕQ?/span>Õø?/span>3aÕQ?/span>ŃĆ?/span>822 cm-1ÕżäńÜäÕ│░ÕĮÆÕøĀõ║ÄõĖāÕŚ¬ńÄ»ńÜäõ╝ĖńŠāµī»ÕŖ©ÕQ?/span>886 cm-1ÕżäńÜäÕ│░ÕĮÆÕøĀõ║ÄN-Hķö«ńÜäÕ╝»µø▓µī»ÕŖ©ŃĆ?/span>Õ£?/span>1489ÕÆ?/span>1710 cm-1ķÖäĶ┐æńÜäÕ│░ÕłåÕł½Õ»╣Õ║öõ║ĵØéńÄ»õĖŁńÜ?/span>-C=NÕÆ?/span>N-C=NÕQīĶĆīÕ£©1338 cm-1ķÖäĶ┐æńÜäÕ│░ÕłÖµØźµ║Éõ║Ä-CNńÜäõÄūŠ~®ŃĆéķÜÅńØĆÕģēńģ¦µŚēÖŚ┤ńÜäÕó×ÕŖĀ’╝īÕ│?/span>ńÜ?/span>Õ╝║Õ║”µśÄµśŠÕó×Õż¦ÕQīÕ│░õĮŹńĮ«õ┐صīüõĖŹÕÅśŃĆéĶ┐Öõ║øń╗ōµ×£ĶĪ©µśÄ’╝ī1.0%-Pt/CN-BH-HµĀĘÕōüõĖ?/span>g-C3N4ńÜäń╗ōµ×äÕÆīÕī¢ÕŁ”ķö«Õ£©ÕÅ»Ķ¦üÕģ?/span>ńģ¦Õ░äõĖŗńö▒õ║ÄÕ╝║ńāłńÜäńö?sh©┤)ÕŁÉõ╝ĀķĆÆĶĆīÕÅæńö¤µśÄµśæųÅśÕī?/span>ŃĆéõžō(f©┤)õ║åµ»öĶŠā’╝īµłæõ╗¼śqśńĀöĮIČõ║å1.0%-Pt/CN-PµĀĘÕōüÕQ?/span>Õø?/span>3bÕQ?/span>ŃĆ?/span>1.0%-Pt/CN-Pµ▓Īµ£ēµśÄµśŠńÜäÕ│░ńÜ?/span>ÕÅ?/span>Õī?/span>ÕQīĶ┐ÖÕÅ»ĶāĮµś»ńö▒õ║?/span>Pt2 õĖ?/span>g-C3N4õ╣ŗķŚ┤ńÜäńöĄ(sh©┤)ÕŁÉĶØ{┐U╗ĶāĮÕŖøĶŠāÕĘ«µēĆĶć┤ŃĆéĶ┐Öõ║øń╗ōµ×£ĶĪ©µśÄ’╝īķ½?/span>Pt0µ»öõŠŗµ£ēÕł®õ║ÄńöĄ(sh©┤)ĶŹ’L(f©źng)ÜäÕłåń”╗ÕÆīõ±öµ░óµ┤╗µĆ¦ńÜäµÅÉķ½śŃĆ?/span>
õĖ▐Z║åµÅŁńż║1.0%-Pt/CN-BH-HõĖ?/span>Pt0ńÜäÕ┼×µłÉµ£║ńÉå’╝īķććńö©ÕĤõĮŹŠUóÕż¢ÕģēĶ░▒µ©Īµŗ¤õ║?/span>1.0%-Pt/CN-BHńÜäµ░óµ░«µž£ÕÉ?/span>µ░öµ░øÕżäńÉåśqćń©ŗŃĆéÕ”éÕø?/span>3cÕÆ?/span>3dµēĆĮC║’╝īC-NµØéńÄ»õĖ?/span>C-Nķö«ńÜäÕ│░Õ£©1200-1750 cm-1ĶīāÕø┤ÕåģµśŠĶæŚÕó×ÕŖĀŃĆéķÜÅńØĆńäÖńā¦µĖ®Õ║”ńÜäÕŹćķ½ś’╝īC-Nķö«ńÜäµī»ÕŖ©µ©ĪÕ╝ÅÕÅæńö¤µö╣ÕÅśÕQ?/span>1710 cm-1ÕżäńÜäÕ│░Õ╝║Õ║”ķĆɵĖÉÕó×Õ╝║ÕQīĶĪ©µś?/span>C3N4Šlōµ×äõĖ?/span>NÕģāń┤ĀńÜäńöĄ(sh©┤)Ķ┤¤µĆ¦ÕÅæńö¤µö╣ÕÅ?/span>ÕQ?/span>Õø?/span>3dÕQ?/span>ŃĆéĶ┐Öõ║øń╗ōµ×£Ķ»üÕ«×õ║åÕ£©µ░öµ░?/span>ÕżäńÉåśqćń©ŗõĖŁ’╝īÕĮōńöĄ(sh©┤)ÕŁÉõ╗ÄNÕģāń┤ĀĶĮ¼ń¦╗Õł?/span>PtµŚė×╝īÕż¦ķćÅńÜ?/span>Pt2 ĶĮ¼ÕÅśõĖ?/span>Pt0ńē®ń¦ŹŃĆ?/span>ÕøĀµŁżÕQīµłæõ╗¼ÕÅ»õ╗źµÄ©µ¢?/span>1710 cm-1ÕżäÕ│░Õ╝?/span>ńÜäÕÅśÕī¢µś»ńöūā║ÄC-Nķö?/span>ķö«ĶāĮńÜäÕÅśÕī¢’╝īĶĪ©µśÄg-C3N4Šlōµ×äõĖ?/span>NÕģāń┤Āńö?sh©┤)Ķ┤¤µĆ¦ńÜäÕÅśÕī¢ŃĆéĶ┐Öõ║øń╗ōµ×£Ķ»üÕ«?/span>Õ£?/span>µ░öµ░øńäÖńā¦śqćń©ŗõĖŁ’╝īÕż¦ķćÅńÜ?/span>Pt2 ķĆÜĶ┐ćNÕģāń┤ĀńÜäÕÉĖÕ╝ĢńöĄ(sh©┤)ÕŁÉĶØ{Õī¢õžō(f©┤)Pt0ńē®ń¦ŹŃĆ?/span>
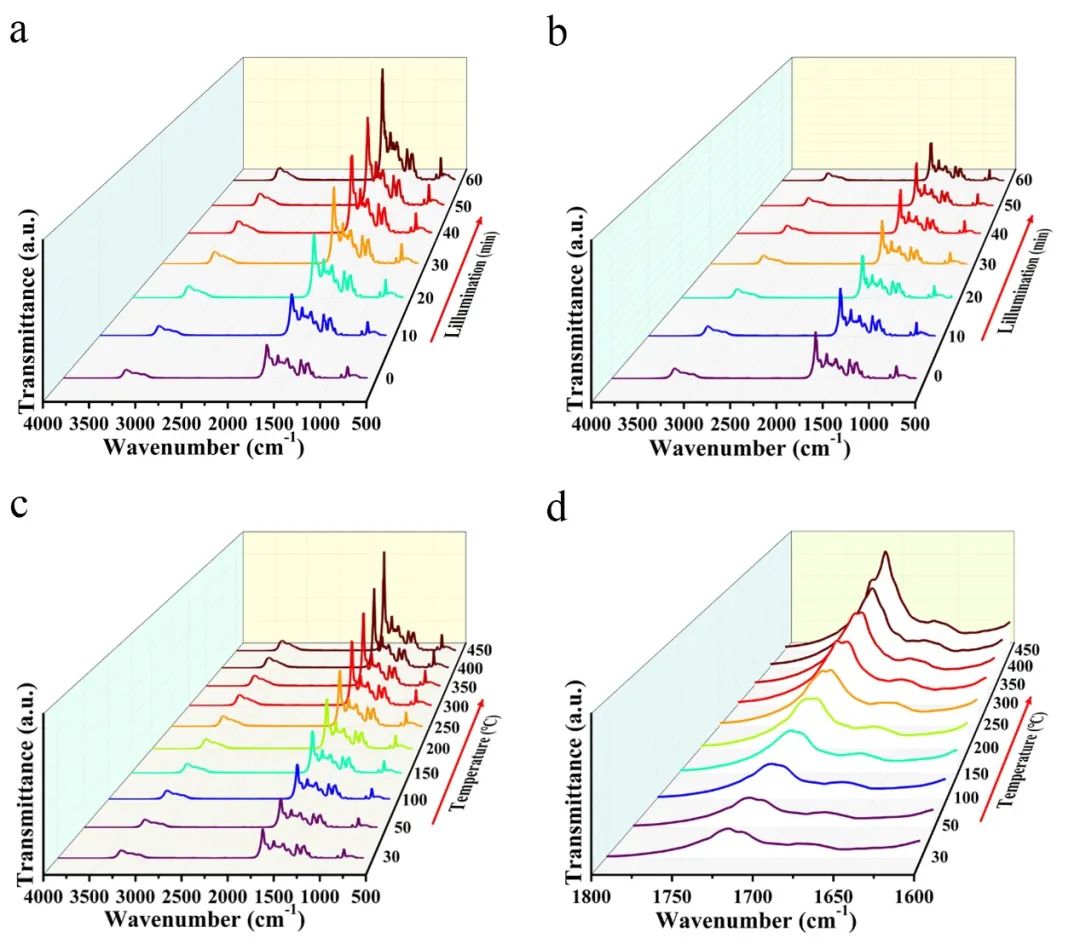
Õø?/span>3 ÕĤõĮŹŠUóÕż¢ĶĪ©ÕŠüŃĆ?/span>ÕQ?/span>aÕQ?/span>1.0%-Pt/CN-BH-HÕÆī’╝łbÕQ?/span>1.0%-Pt/CN-PÕ£©ÕÅ»Ķ¦üÕģēÕQ?/span>╬╗ Ōē?420 nmÕQ?/span>ńģ¦Õ░äõĖŗńÜäÕĤõĮŹŠUóÕż¢ÕģēĶ░▒ŃĆ?/span>ÕQ?/span>cÕQ?/span>1.0%-Pt/CN-BHÕ£©µ░öµ░øÕżäńÉ?/span>ÕÆīÕŖĀńā?/span>śqćń©ŗõĖŁńÜäÕĤõĮŹŠUóÕż¢ÕģēĶ░▒ÕÆ?/span>ÕQ?/span>dÕQ?/span>Õ▒Ćķā©µöŠÕż¦ÕøŠŃĆ?/span>
õĖ▐Z║åśqøõĖƵŁźńĀöĮI?/span>Ptńē®ń¦ŹńÜäÕ¬äÕōŹ’╝īµłæõ╗¼Õ╗║ń½ŗõ║?/span>PtõĖ?/span>g-C3N4ÕQ?/span>Õø?/span> 4aÕÆ?4dÕQ?/span>õ╣ŗķŚ┤ńÜäõ╝śÕī¢ń╗ōµ×䵩ĪÕ×ŗŃĆéĶ»źµ©ĪÕ×ŗõ╗ŻĶĪ©õ║åõĖŹÕÉīń╗ōµ×䵩ĪÕ×ŗÕ»╣Õ║öńÜäPtńÜäõĖŹÕÉīķģŹõĮŹµ©ĪÕ╝ÅÕÆīńŖȵĆü’╝īÕīģµŗ¼Pt2 ÕÆ?/span>Pt0ńē®ń¦ŹŃĆéńöĄ(sh©┤)ĶŹĘÕ»åÕ║”ÕĘ«ÕøŠµśŠĮC▐Z║åńö?sh©┤)ÕŁÉÕ»åÕ║”ńÜäÕż¦×«Å’╝īÕ”éÕø?/span>4bÕÆ?/span>4eµēĆĮC║ŃĆéµĀ╣µŹ?/span>Baderńö?sh©┤)ĶŹĘÕłåµ×ÉÕQīÕ£©Pt2 ńē®ń¦ŹÕŁśÕ£©õĖŗ’╝īńö?sh©┤)ÕŁÉõ?/span>PtÕÉ?/span>g-C3N4ĶĮ¼ń¦╗ÕQīĶØ{┐U╗ķćÅõĖ?/span>1.24ŃĆéÕĮōÕŁśÕ£©Pt0ńē®ń¦ŹµŚė×╝īńö?sh©┤)ÕŁÉõ?/span>g-C3N4ĶĮ¼ń¦╗Õł?/span>PtÕQīĶØ{┐U╗ķćÅõĖ?/span>0.68ŃĆéĶ┐Öõ║øń╗ōµ×£ĶĪ©µśÄ’╝īPt2 ńē®ń¦ŹńÜäÕŁśÕ£©õĖŹÕł®õ║Äńö?sh©┤)ÕŁÉńÜ䵏ĢĶÄŚ„Ć?/span>Õ£?/span>1.0%-Pt/CN-BH-HµĀĘÕōüõĖ?/span>ÕQ?/span>ńöūā║ÄPt0ńē®ń¦Źµ»öõŠŗĶŠāķ½śÕQīõĖöPt0ńÜ?/span>ńö?/span>µłÉµÅÉķ½śõ║åńö?sh©┤)ĶŹĘÕłåń”╗µĢłńÄćÕQ?/span>g-C3N4õĖŖńÜäńö?sh©┤)ÕŁÉµø┤ķ½śµĢłÕ£░ĶĮ¼ń¦╗Õ?/span>PtõĖŖŃĆ?/span>PtÕ£©õĖŹÕÉīÕī¢ÕŁ”ńŖȵĆüõĖŗÕ»ęÄ(gu©®)░óµ░öńÜäÕÉöRÖäĶāĮÕ”éÕø?/span>4cÕÆ?/span>4fµēĆĮC║ŃĆ?/span>ķĆÜĶ┐ćĶ«Īń«ŚÕŠŚÕł░Pt2 ÕÆ?/span>Pt0ńÜäÕÉĖķÖäĶāĮÕłåÕł½õĖ?/span>-0.105ÕÆ?/span>-0.098 eVŃĆ?/span>Pt2 ÕÆ?/span>Pt0ńÜäÕÉĖķÖäĶĘØ╝øšdłåÕł½õžō(f©┤)2.63ÕÆ?/span>2.79 ?ŃĆéńö▒õ║?/span>Pt0ńē®ń¦ŹÕģõh£ēĶŠāõĮÄńÜäÕÉĖķÖäĶāĮÕQīÕ£©Õɽµ£ēPt0ńē®ń¦ŹńÜäÕé¼Õī¢ÕēéõĖŁ’╝īµ░?/span>µ░?/span>µø┤Õ«╣µśōµ║óÕć║ŃĆéÕøĀµŁż’╝īµłæõ╗¼ÕÅ»õ╗źÕŠŚÕć║Õó×ÕŖĀPt0ńē®ń¦ŹÕɽķćŵ£ēÕł®õ║ĵ×ɵ░óŃĆéĶ┐ÖõĖĆŠlōµ×£õ╣¤õĖÄõĖŖĶ┐░ÕĤõĮŹŠUóÕż¢ÕģēĶ░▒ńÜäń╗ōĶ«▐ZĖĆĶć┤ŃĆ鵣żÕż¢’╝īśqśĶ»┤µśÄõ║åPt0ńē®ń¦ŹÕ£©ÕģēÕé¼Õī¢ÕłČµ░óõĖŁńÜäõ╝śĶČŖµĆ¦ŃĆ?/span>
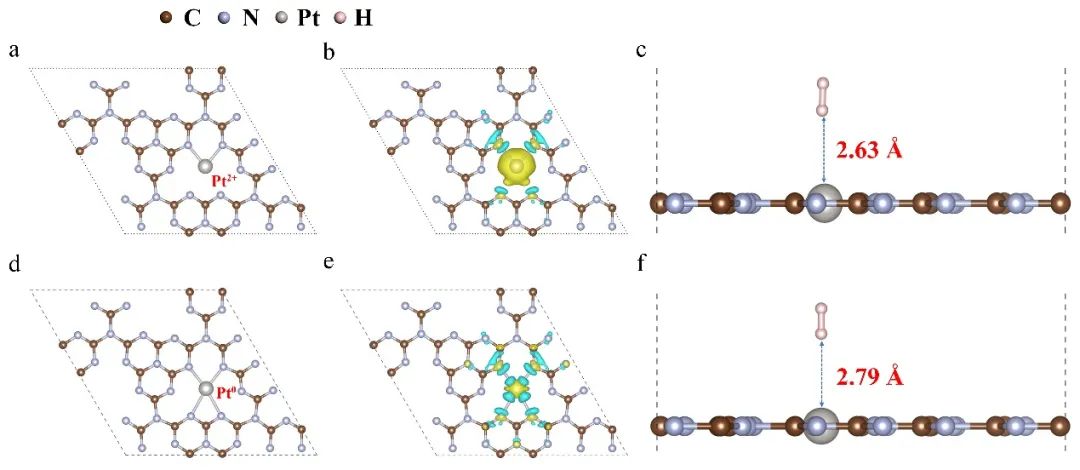
Õø?/span>4 DFTńÉåĶ«║Ķ«Īń«ŚŃĆé’╝łaÕQ?/span>dÕQ?/span>õ╝śÕī¢Šlōµ×äÕQ?/span>ÕQ?/span>bÕQ?/span>eÕQ?/span>ÕĘ?/span>Õł?/span>ńö?sh©┤)ĶŹĘÕ»åÕ║”Õ?/span>ÕQ?/span>cÕQ?/span>fÕQ?/span>Pt2 ÕÆ?/span>Pt0ńē®ń¦ŹÕ»ęÄ(gu©®)░óµ░?/span>ńÜäÕÉĖķÖäĶāĮŃĆ?/span>
µ£¼ńĀöĮIȵłÉÕŖ¤ÕłČÕżćõ║åķ½?/span>Pt0µ»öõŠŗÕQ?/span>60.1%ÕQ?/span>ńÜäÕģēÕé¼Õī¢ÕēéŃĆ?/span>Õ«āńÜäÕģēÕé¼Õī¢õ±öµ░óķƤńÄćĶŠæųł░2.316 mmol h-1 g-1ÕQīµ»öÕÅ»Ķ¦üÕģēńģ¦×«?/span>ÕQ?/span>╬╗ Ōē?420 nmÕQ?/span>õĖ?/span>Pt0µ»öõŠŗĶŠāõĮÄÕQ?/span>8.2%ÕQ?/span>ńÜ?/span>1.0%-Pt/CN-PÕQ?/span>0.605 mmol h-1 g-1ÕQ?/span>µÅÉķ½śõ║?/span>4ÕĆŹŃĆ?/span>1.0%-Pt/CN-BH-HńÜäķ½śÕģēÕé¼Õī¢µĆ¦ĶāĮÕÅ»ÕĮÆÕøĀõ║ÄÕģČõĖŁÕɽµ£ēÕż¦ķćÅńÜ?/span>Pt0ńē®ń¦ŹÕQīÕŖĀķƤõ║åÕģēńö¤ńö?sh©┤)ĶŹĘńÜäÕłå╝?/span>ŃĆ?/span>ÕÅ”Õż¢ÕQ?/span>Pt0ńē®ń¦ŹĶŠāõĮÄńÜäÕÉĖķÖäĶāĮµ£ēÕł®õ║ĵ░óµ░öńÜäµ║óÕć║ŃĆéÕøĀµŁż’╝īĶ░āµÄ¦Õī¢ÕŁ”ńŖȵĆüÕÅ»ĶāĮµś»Õ╝ĆÕÅæµ¢░Õ×ŗÕģēÕé¼Õī¢ÕēéńÜäµ£ēµĢłĮ{¢ńĢźŃĆ?/span>
Ķ«║µ¢ćĮW¼õĖĆõĮ£ĶĆģõžō(f©┤)õĖŁÕøĮń¤Žxča(b©│)Õż¦ÕŁ”ÕQłÕīŚõ║¼’╝ēÕŹÜÕŻ½ńö¤µŁ”õĮŽxś¤ÕQīĶ«║µ¢ćķĆÜĶ«»õĮ£ĶĆģõžō(f©┤)õĖŁÕøĮń¤Žxča(b©│)Õż¦ÕŁ”ÕQłÕīŚõ║¼’╝ēńÄŗķøģÕÉøÕē»ńĀöń®ČÕæśÕÆīµĖģÕŹÄÕż¦ÕŁ”µ£▒µ░Ėµ│ĢµĢֵijŃĆ鵣żńĀöń®ČÕŠŚÕł░ÕøĮÕ«ČķćŹńé╣ńĀöÕÅæĶ«ĪÕłÆĮ{ēĶĄäÕŖ®µö»µīüŃĆ?/span>
ÕĤµ¢ćķōŠµÄź
https://www.sciencedirect.com/science/article/pii/S1385894722018290
ÕģŹĶ┤ŻÕŻ░µśÄÕQÜķā©ÕłåĶĄäµ¢ÖµØźµ║Éõ║ÄŠ|æń╗£ÕQīĶØ{ĶĮĮńÜäńø«ńÜäÕ£©õ║Äõ╝ĀķĆƵø┤ÕżÜõ┐Īµü»ÕÅŖ(qi©óng)ÕłåõĒnÕQīÕŲłõĖŹµäÅÕæ│ńØĆĶĄ×ÕÉīÕģČĶ¦éńéęÄ(gu©®)ł¢Ķ»üÕ«×ÕģČń£¤Õ«×µĆ¦’╝ī
õ╣¤õĖŹµ×䵳ÉÕģČõ╗¢Õ╗°Ö««ŃĆéõ╗ģµÅÉõŠøõ║żµĄü“qø_Å░ÕQīõĖŹõĖ║ÕģČńēłµØāĶ┤¤Ķ┤ŻŃĆéÕ”éµČēÕÅŖ(qi©óng)õŠē|ØāÕQīĶ»ĘĶüöń│╗µłæõ╗¼ÕÅ?qi©óng)µŚČõ┐«µö╣µł¢ÕłĀķÖżŃĆ?br>ķé«ń«▒ÕQÜinfo@polymer.cn
ÕQłĶ┤Żõ╗╚Ø╝¢ĶŠæ’╝Ü(x©¼)sunÕQ?
- ńøĖÕģ│µ¢░ķŚ╗
- µŚĀńøĖÕģŽx¢░ķŚ?/li>



